Bone tissue is largely considered an inert mineral reservoir that provides a framework for locomotion and physical strength. However, if we drill down into the origins of bone cells, we find that the interconnected bone and immune cells’ crosstalk plays a key role in not only bone mineral metabolism and bone remodeling but inflammatory signaling that can contribute to bone loss. In this article, we will review osteoimmunological interactions and nutraceutical options that are emerging as novel bone health options.
The Bone-Immune Connection
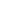
Bone is primarily comprised of three cell types: osteoclasts (OC), osteoblasts (OB) and osteocytes. The outer layer of compact bone surrounds the inner layer of trabecular spongy bone that contains pores housing red and white bone marrow. The inner matrix of bone not only gives rise to red and white blood cells (WBCs), but also precursor stem cells to OBs, OCs and immune cells. The heart of the bone-immune connection is in the shared lineage of stem cells and shared signaling pathways. Both bone cells and the immune system have a bidirectional influence on each other. Inflammatory bone loss in autoimmune conditions highlight this outside-in connection. The influence of OBs and OCs on immune cell generation, with defects in OCs and OBs causing anemia and infection, highlights the inside-out connection.
The receptor activator of nuclear factor kappa-? ligand (RANKL), receptor activator of nuclear factor kappa-? ligand (RANK) and osteoprotegerin (OPG) system, or RANKL/RANK/OPG axis, is a critical inflammatory signaling pathway in bone-immune cross-communication, with the potent RANKL cytokine and receptor (RANK) as the signal initiators. OPG acts as a receptor decoy that keeps this signaling pathway in balance (diagram).
Image source: Richards, J. Brent, Hou-Feng Zheng, and Tim D. Spector. "Genetics of osteoporosis from genome-wide association studies: advances and challenges." Nature Reviews Genetics 13.8 (2012): 576-588.
Chronic inflammation and oxidative stress are stimulators of RANKL and cytokine production in general, and also block sufficient OPG production. The RANK signal changes the fate of stem cells destined to become macrophages and monocytes, redirecting them toward osteoclastogenesis. This path causes precursor OCs to fuse and form a catabolically active osteoclast that secretes acid and enzymes for bone destruction.
Bone remodeling must have a balanced cycle of OCs resorbing old bone and OBs building new bone to maintain a healthy, resilient skeleton. When bone resorption outpaces bone building, a pathological state occurs, and bone becomes weak and fragile. Bone resorption is highly influenced by the specific cocktail of pro-inflammatory cytokines and growth factors that signal the cellular differentiation, activation and survival of bone-resorbing osteoclasts.
Given the modern pro-inflammatory diet, lack of bone-building nutrients and drastic rise of chronic disease, inflammatory-driven bone loss will become more prevalent beyond autoimmune conditions. Understanding of the RANKL/RANK/OPG axis presents an opportunity for an updated bone health treatment strategy beyond mineral replacement and vitamin D. In fact, the pharmaceutical denosumab, a monoclonal IgG2 antibody against RANKL, was developed to inhibit RANKL and ameliorate excessive bone resorption by suppressing osteoclastogenesis.
Nutraceutical Intervention
Many nutrients provide anti-inflammatory and antioxidant benefits that may also play a role in downregulating cytokine production, RANKL production and dampening the immune response to preserve and build bone.
Fish oil
Past studies have indicated a positive correlation between the intake of omega-3 fatty acids and bone mineral density in postmenopausal women. Recent research has pointed to eicosapentaenoic (EPA) and docosahexaenoic (DHA) acids to reduce the number of osteoclasts and activity by suppressing RANK and RANKL expression along with other potent cytokines TNFa, IL-6 and PGE2. Finally, lipid mediators from omega-3 fatty acids have also shown bone preservation effects by modulating RANKL and OPG expression. The preliminary evidence is showing fish oil to be a strong option for balancing bone-immune crosstalk.
NAC
N-acetyl cysteine (NAC) is a powerful antioxidant that has shown reduce oxidative stress, boost glutathione synthesis and reduce inflammation. In a postmenopausal osteoporosis animal model, NAC was found to prevent estrogen-deficient mice from bone loss by inhibiting oxidative stress, DNA damage and cellular senescence. Obesity has also shown to increase bone resorption due to increased cytokine production. Animal models where mice were fed high-fat diets and supplemented with NAC were shown to have higher glutathione status, decreased osteoclast formation and decreased bone resorption.
Selenium
Selenium is an essential trace element that has shown to improve bone health, immune surveillance, protect cell growth and acts as a potent antioxidant. Excessive intracellular reactive oxygen species (ROS) contribute to the development of osteoporosis by blocking osteoblasts developing from stem cells. Selenium increases the production of glutathione peroxidases, thioredoxin reductases and selenoproteins, which play key roles in reducing intracellular ROS and protecting osteoblasts and balancing immune function. A recent study from China also found an association between dietary intake of selenium and osteoporosis risk.
Turmeric
A recently published study at the University of Arizona College of Medicine shows turmeric may be an effective phytonutrient for preventing osteoporosis and bone loss. The turmeric extracts used in the study prevented trabecular bone loss and bone mineral density loss in the spine and hips, suggesting a reduced fracture risk from turmeric. A study performed on 100 bedridden spinal cord injury patients studied curcumin’s effects on bone turnover markers and BMD in the lumbar spine, hip and femoral neck of patients. At six months, biomarkers and BMD indicators suggested a decrease in the progress of osteoporosis. Another study performed on 57 osteopenic patients with low bone density who were given curcumin noted an increase in bone density readings after 24 weeks.
Quercetin
Quercetin is a major dietary flavonoid found in onions and other vegetables that has shown benefits for many diseases and recently for bone loss. An animal model of postmenopausal osteoporosis showed that were given quercetin in their diet improved higher BMD in the lumbar spine and femur. Scientists also performed in vitro experiments and found a dose-dependent reduction of RANK/RANKL-stimulated expression of osteoclasts, building a strong case for quercetin as a potent inhibitor of osteoclastogenesis, and possible selective estrogen receptor modulator.

Dr. Frank Bodnar is a licensed chiropractor in the states of Wisconsin and Illinois. He holds a doctorate of chiropractic from Palmer College of Chiropractic, and Master of Science degree (magna cum laude) in human nutrition from the University of Bridgeport.
He is certified in sports nutrition from the International Society of Sports Nutrition (CISSN), and is a certified CrossFit Trainer (CF-L1).
References
- Ponzetti M, Rucci N. Updates on Osteoimmunology: What's New on the Cross-Talk Between Bone and Immune System. Front Endocrinol (Lausanne). 2019 Apr 18;10:236.
- Guder C, Gravius S, Burger C, Wirtz DC, Schildberg FA. Osteoimmunology: A Current Update of the Interplay Between Bone and the Immune System. Front Immunol. 2020 Jan 31;11:58.
- M. Neale Weitzmann, "The Role of Inflammatory Cytokines, the RANKL/OPG Axis, and the Immunoskeletal Interface in Physiological Bone Turnover and Osteoporosis", Scientifica, vol. 2013, Article ID 125705, 29 pages, 2013.
- Ono T, Hayashi M, Sasaki F, Nakashima T. RANKL biology: bone metabolism, the immune system, and beyond. Inflamm Regen. 2020 Feb 7;40:2. doi: 10.1186/s41232-019-0111-3.
- Orchard, T. S., Pan, X., Cheek, F., Ing, S. W., & Jackson, R. D. (2012). A systematic review of omega-3 fatty acids and osteoporosis. The British journal of nutrition, 107 Suppl 2(0 2), S253–S260.
- Nakanishi, A., Iitsuka, N., & Tsukamoto, I. (2013). Fish oil suppresses bone resorption by inhibiting osteoclastogenesis through decreased expression of M-CSF, PU.1, MITF and RANK in ovariectomized rats. Molecular Medicine Reports, 7, 1896-1903.
- Kajarabille N, Díaz-Castro J, Hijano S, López-Frías M, López-Aliaga I, Ochoa JJ. A new insight to bone turnover: role of ?-3 polyunsaturated fatty acids. ScientificWorldJournal. 2013 Nov 4;2013:589641.
- El Kholy, K., Freire, M., Chen, T., & Van Dyke, T. E. (2018). Resolvin E1 Promotes Bone Preservation Under Inflammatory Conditions. Frontiers in immunology, 9, 1300.
- Zhou, X., Wang, Z., Ni, Y., Yu, Y., Wang, G., & Chen, L. (2020). Suppression effect of N-acetylcysteine on bone loss in ovariectomized mice. American journal of translational research, 12(3), 731–742.
- Jay J. Cao, Matthew J. Picklo, N-Acetylcysteine Supplementation Decreases Osteoclast Differentiation and Increases Bone Mass in Mice Fed a High-Fat Diet, The Journal of Nutrition, Volume 144, Issue 3, March 2014, Pages 289–296
- Zeng, H., Cao, J. J., & Combs, G. F., Jr (2013). Selenium in bone health: roles in antioxidant protection and cell proliferation. Nutrients, 5(1), 97–110.
- Wang Y, Xie D, Li J, Long H, Wu J, Wu Z, He H, Wang H, Yang T, Wang Y. Association between dietary selenium intake and the prevalence of osteoporosis: a cross-sectional study. BMC Musculoskelet Disord. 2019 Dec 4;20(1):585.
- Wright LE, Frye JB, Timmermann BN, Funk JL. Protection of trabecular bone in ovariectomized rats by turmeric (Curcuma longa L.) is dependent on extract composition. J Agric Food Chem. 2010 Sep 8;58(17):9498-504.
- Hatefi M, Ahmadi MRH, Rahmani A, Dastjerdi MM, Asadollahi K. Effects of Curcumin on Bone Loss and Biochemical Markers of Bone Turnover in Patients with Spinal Cord Injury. World Neurosurg. 2018 Jun;114:e785-e791.
- Riva A, Togni S, Giacomelli L, Franceschi F, Eggenhoffner R, Feragalli B, Belcaro G, Cacchio M, Shu H, Dugall M. Effects of a curcumin-based supplementation in asymptomatic subjects with low bone density: a preliminary 24-week supplement study. Eur Rev Med Pharmacol Sci. 2017 Apr;21(7):1684-1689.
- Wong SK, Chin KY, Ima-Nirwana S. Quercetin as an Agent for Protecting the Bone: A Review of the Current Evidence. Int J Mol Sci. 2020 Sep 3;21(17):6448.
- Huang, Y. Y., Wang, Z. H., Deng, L. H., Wang, H., & Zheng, Q. (2020). Oral Administration of Quercetin or Its Derivatives Inhibit Bone Loss in Animal Model of Osteoporosis. Oxidative medicine and cellular longevity, 2020, 6080597.
- Tsuji M, Yamamoto H, Sato T, Mizuha Y, Kawai Y, Taketani Y, Kato S, Terao J, Inakuma T, Takeda E. Dietary quercetin inhibits bone loss without effect on the uterus in ovariectomized mice. J Bone Miner Metab. 2009;27(6):673-81.