Most clinicians are familiar with autoimmune disease mechanisms. Typically, these define situations where effectors within the adaptive immune system (i.e., immunoglobulins or T-cell receptors) bind inappropriately to a host molecule which subsequently recruits an immune response against those tissues. Processes of self-tolerance, whereby naïve B and T lymphocytes are exposed to self-antigen in a controlled environment, are used to find cells producing cross-reactive antibodies or T-cell receptors so they can be eliminated before they circulate in the body. When these cells are not removed properly, or unprocessed antigens have unfettered access to the immune system (perhaps through breaches in the gut barrier), they can proliferate and produce memory cells that trigger years of intermittent attacks on host tissues and cells (i.e., autoimmune reactivity).
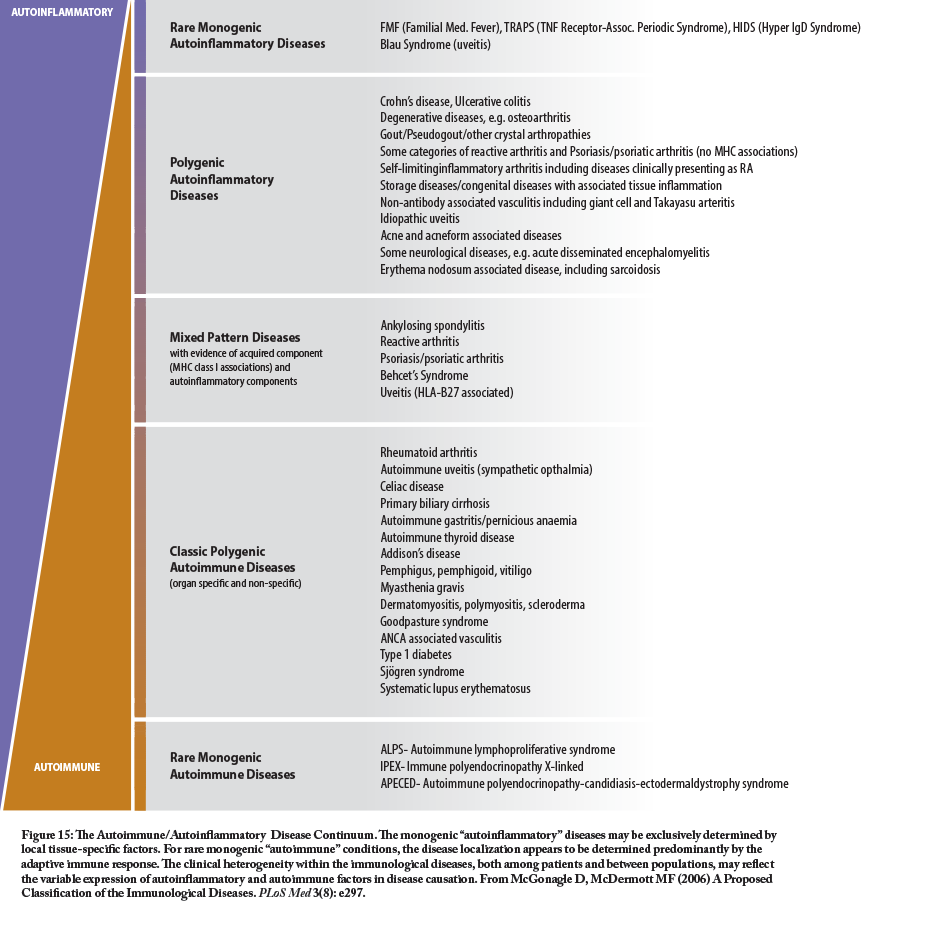
However, the past several decades have seen a much greater understanding of the innate immune system and its role in chronic immune-related dysfunctions, especially those related to inflammation. Some of these conditions have strong similarities to, or co-exist with, auto-immune conditions and are generally classified as auto-inflammatory conditions. The classic autoinflammatory syndromes are usually related to monogenetic or polygenetic mutations in genes that are important in the regulation of the innate immune response (See http://www.nomidalliance.org/ for more information). The chart shows the relationship and overlap of some of the more common autoinflammatory and autoimmune conditions.
While each of these conditions has unique therapeutic considerations based on their manifestations and target tissues, each can be complimented with a global anti-inflammatory strategy. Such an approach mandates the incorporation of lifestyle-related therapies such as an anti-inflammatory diet, reduced HPA axis stress, moderate physical activity, adequate and timely sleep patterns, and the avoidance of environmental toxins that exacerbate the need for detoxification and depletes antioxidant reserves. Clinicians should have intervention strategies available for each of these areas of lifestyle intervention if they are to adequately address either autoinflammatory or autoimmune conditions. In addition, there are numerous supplemental nutrients that can diminish or resolve the inflammatory process that should be considered when treating patients with chronic inflammatory conditions; many of these supplemental nutrients have positive clinical outcomes related to autoinflammatory syndromes or their mechanisms. Such nutrients include omega-3 fatty acids, vitamin D, curcumin, quercetin, boswellia, propolis (or CAPE), Scutellaria biacalensis (Chinese skullcap) and ginger. These and other compounds designed to modulate immune and GI function (remember that barrier function is key to a balanced immune response) will be invaluable for what appears to be a growing number of autoinflammatory cases.
About Thomas G. Guilliams Ph.D.

Dr. Guilliams earned his doctorate from the Medical College of Wisconsin (Milwaukee) where he studied molecular immunology in the Microbiology Department. Since 1996, he has spent his time studying the mechanisms and actions of natural-based therapies, and is an expert in the therapeutic uses of nutritional supplements. As the Vice President of Scientific Affairs for Ortho Molecular Products, he has developed a wide array of products and programs which allow clinicians to use nutritional supplements and lifestyle interventions as safe, evidence-based and effective tools for a variety of patients. Tom teaches at the University of Wisconsin-School of Pharmacy, where he holds an appointment as a Clinical Instructor; at the University of Minnesota School of Pharmacy and is a faculty member of the Fellowship in Anti-aging Regenerative and Functional Medicine. He lives outside of Stevens Point, Wisconsin with his wife and children.