Chronic pain is a widespread, incapacitating and expensive condition. In the United States alone, an estimated 126 million adults reported some type of pain in the previous three months, with 25.3 million adults (11.2%) reporting daily (chronic) pain and 23.4 million (10.3%) describing intense pain.1 Chronic pain costs the United States an estimated $560 to $635 billion annually.1
The increasing prevalence of the link between chronic pain and opioid abuse has been described as an epidemic. In 2015, there were 33,091 deaths involving unintentional opioid overdose. Most of these individuals were first exposed to an opioid for the treatment of acute or chronic pain.2
A high proportion of primary care visits involve, at least in part, the management of chronic pain.3 Low back pain is the leading cause of years lived with disability both in the United States and globally and accounts for one-third of all work loss. Of the top nine leading causes of disability, four are musculoskeletal, in low back pain, neck pain, osteoarthritis and migraine headaches.4
If there is one big idea that we can all agree on, based on this information, it’s that the treatment of pain in the past was not as effective as we thought, and we need innovative treatment strategies moving forward. The complexity of pain needs a multidimensional approach. Addressing hidden drivers of inflammation such as stress, a damaged gastrointestinal tract and obesity are just a few examples5-7 of targets that are modifiable via nutritional therapy and can result in decreased systemic inflammation—the culprit in many painful chronic conditions.
Case Study
An 82-year-old male presents with severe pain in both anterior-lateral thighs and lateral shoulders, rated at 7/10 on the Visual Analogue Scale (VAS). He reports that the pain is constant and only mild relief is found with over-the-counter (OTC) non-steroidal anti-inflammatory drugs (NSAIDs) and gabapentin. The patient is severely limited in most of his very basic activities of daily living (ADLs) and is unable to walk without a cane or walker. Even with assistance, he must stop every 20 yards to catch his breath and regain his balance. His balance issues are compounded by severe peripheral neuropathy in his legs for which he was diagnosed by his primary care physician about six years ago. He reports intense brain fog and forgetfulness; he can drive a car but avoids it because doing so makes him very nervous. The patient has multiple co-morbidities and was diagnosed with hypercholesterolemia 25 years ago and put on a statin medication. About a decade later, he was diagnosed with type 2 diabetes and put on metformin and has since added an insulin drug as well. Although he followed medication directions, his diabetes progressed, his hemoglobin A1c (HbA1c) rose to above 9 and he was put on 50 units of insulin per day. His pain, which began mildly as aches in his legs about 15 years ago, grew into inexorable pain in his legs and shoulders over the next six years. He was prescribed NSAIDs and gabapentin, which did provide mild relief. He also noticed that his dizziness and loss of balance became more severe as his condition progressed.
Treatment and Outcome
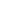
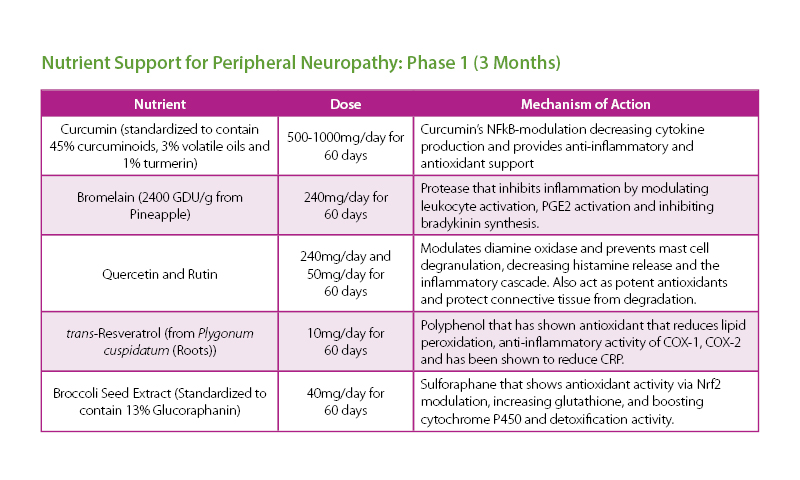
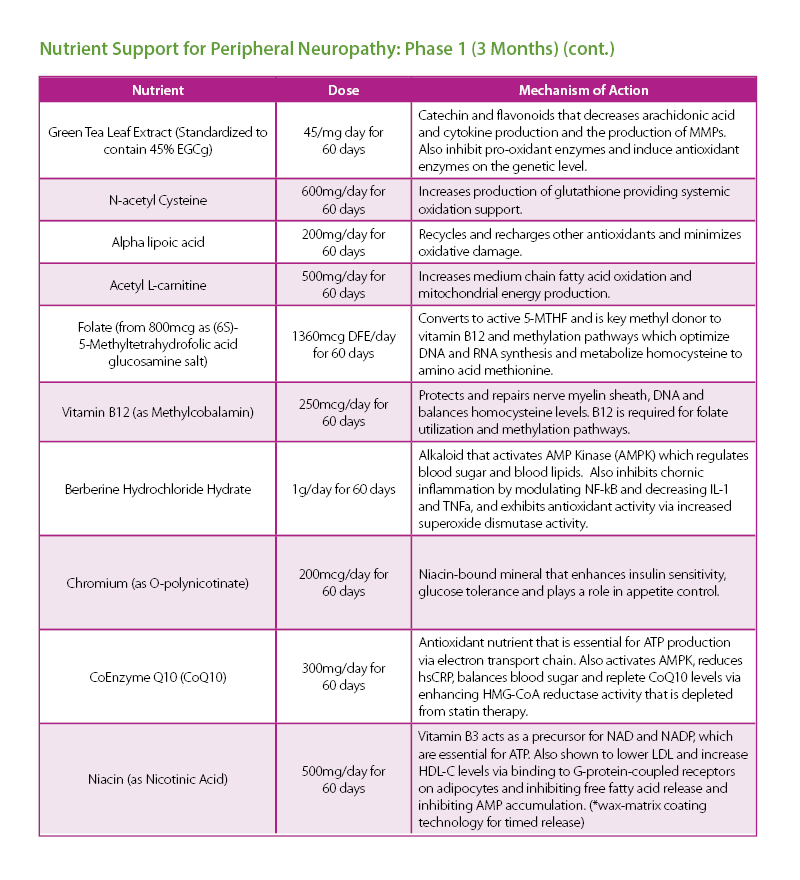
The patient was given a treatment plan that included chiropractic adjustments, exercise rehabilitation exercises and a walking and biking plan for the next three months. He was also prescribed a time-restricted Paleo diet, which he followed strictly for three months. He met with a health coach on a weekly basis for accountability. Finally, the patient was also prescribed a broad-spectrum nutraceutical regimen that targeted the pleotropic causes of metabolic dysfunction, inflammation and filled his drug-induced nutrient depletions.
The results were improved metabolic labs in decreased A1c, LDL cholesterol, fasting blood sugar and weight loss of over 30 lbs. The patient was able to lower his statin medication and discontinue his insulin.
Discussion
The factors leading to diabetic neuropathy are not completely elucidated, and although multiple hypotheses are being explored, there are still a lot of unknowns. We do know that the development of neuropathy is a multifactorial process that depends on impaired glucose tolerance, mitochondrial function of sensory neurons in the dorsal root ganglion, inflammatory cytokine levels, overall oxidative stress burden, genetics, lipid levels, blood pressure, smoking and alcohol use, and exposure to toxins.8,9 Newer research is also pointing to possible autoimmune and vascular injury mechanisms, and we are finding key differences between type 1 (poor glycemic control and loss of nerve conduction) and type 2 (more changes in lipid metabolism) diabetic neuropathies.10,11
In this case, a two-phase plan was developed that would first address the most immediate causes of the patient’s pain via neuropathic damage and secondly, address the oxidative stress and inflammation. The dysfunction of mitochondria in the muscles and the nerves can lead to aberrant cellular signaling coupled with changes in neurotransmission both peripherally and centrally, resulting in sensitization and amplified pain signaling.12 Optimizing glucose metabolism and insulin sensitivity were also primary strategies, but so was reducing oxidative stress and inflammation from multiple sources that can damage nerve myelin, fibers and receptors.13 In this patient, the two primary sources of inflammation were the adipose tissue and gastrointestinal tract. Both can be sources of low-grade chronic inflammation and need to be dampened to lessen further activation of the inflammatory response, cytokine production and pain signaling in the nervous system.14,15 Healing of the gut lining either by building up damaged enterocytes, restoring the mucosal barrier and binding and removing lipopolysaccharides (LPS) should all be considered on an individual basis, and could take anywhere from three to 12 months. Further, the reduction of adipose tissue takes consistent lifestyle changes in dietary and exercise habits that may take significant accountability to improve and will require a similar timeframe in most patients.
ADRIAN DEN BOER DC, ND

Educated in both the Netherlands and the United States, Dr. Adrian den Boer is a board-certified and licensed Naturopathic and Chiropractic physician. In addition, Dr. den Boer is fully certified as a functional medicine doctor. Dr. den Boer has treated over 10,000 patients successfully by utilizing multiple resources to manage patient care. Most recently, he joined the Lifestyle Matrix Resource Center as the Clinical Expert serving the MSK Solutions Pain Recovery Program.
References:
1 Dahlhamer, J., Lucas, J., Zelaya, C., Nahin, R., Mackey, S., DeBar, L., Kerns, R., Von Korff, M., Porter, L. and Helmick, C. (2018). Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults — United States, 2016. [online] Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/mmwr/volumes/67/wr/mm6736a2.htm [Accessed 26 Aug. 2019].
2 Seth, P., Scholl, L., Rudd, R. and Bacon, S. (2018). Overdose Deaths Involving Opioids, Cocaine, and Psychostimulants — United States, 2015–2016. [online] Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/mmwr/volumes/67/wr/mm6712a1.htm [Accessed 26 Aug. 2019].
3 Mills S, Torrance N, Smith BH. Identification and Management of Chronic
Pain in Primary Care: a Review. Curr Psychiatry Rep. 2016;18:22.
4 Mokdad, A., et al. (2018). The State of US Health, 1990-2016. JAMA, [online] 319(14), p.1444. Available at: https://jamanetwork.com/journals/jama/fullarticle/2678018
[Accessed 26 Aug. 2019].
5 de Jong, P., González-Navajas, J. and Jansen, N. (2016). The digestive tract as the origin of systemic inflammation. Critical Care, [online] 20(1). Available at: https://ccforum.biomedcentral.com/articles/10.1186/s13054-016-1458-3 [Accessed 24 Jun. 2019].
6 Marsland, A., Walsh, C., Lockwood, K. and John-Henderson, N. (2017). The effects of acute psychological
stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain, Behavior, and Immunity, [online] 64, pp.208-219. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5553449/ [Accessed 26
Jun. 2019].
7 Adipose tissue inflammation in obesity and metabolic syndrome. (2009). Discovery medicine, [online] Aug;8(41), pp.55-60. Available at: http://www.discoverymedicine.com/Satoshi-Nishimura/2009/09/22/adipose-tissue-inflammation-in-obesity-and-metabolic-syndrome/ [Accessed 24 Jun. 2019].
8 Schreiber, A. (2015). Diabetic neuropathic
pain: Physiopathology and treatment. World Journal of Diabetes, [online] 6(3), p.432. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4398900/ [Accessed 24 Jun. 2019].
9 Said, G. (2019). Diabetic Neuropathy--A Review. [online] Medscape. Available at: https://www.medscape.com/viewarticle/558574_5 [Accessed 24 Jun. 2019].
10 Ellenberg, M. (1974). Diabetic Neuropathic Cachexia. Diabetes, [online] 23(5), pp.418-423. Available at: https://diabetes.diabetesjournals.org/content/23/5/418 [Accessed 24 Jun. 2019].
11 Jende, J., Groener, J., Oikonomou, D., Heiland, S., Kopf, S., Pham, M., Nawroth, P., Bendszus, M. and Kurz, F. (2018). Diabetic neuropathy differs between type 1 and type 2 diabetes: Insights
from magnetic resonance neurography. Annals of Neurology, 83(3), pp.588-598.
12 Areti, A., Ganesh Yerra, V., Komirishetty, P. and Kumar, A. (2016). Potential Therapeutic Benefits of Maintaining Mitochondrial
Health in Peripheral Neuropathies. Current Neuropharmacology, [online] 14(6), pp.593-609. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4981743 [Accessed
24 Jun. 2019].
13 Jin, H. and Park, T. (2018). Role of inflammatory biomarkers in diabetic peripheral neuropathy. Journal of Diabetes Investigation, [online] 9(5), pp.1016-1018. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6123055/ [Accessed 24 Jun. 2019].
14 Adipose tissue inflammation in obesity and metabolic syndrome. (2009). Discovery medicine, [online]
Aug;8(41), pp.55-60. Available at: http://www.discoverymedicine.com/Satoshi-Nishimura/2009/09/22/adipose-tissue-inflammation-in-obesity-and-metabolic-syndrome/ [Accessed 24 Jun. 2019].
15 de Jong, P., González-Navajas, J. and Jansen, N. (2016). The digestive tract as the origin of systemic inflammation. Critical Care, [online] 20(1). Available at: https://ccforum.biomedcentral.com/articles/10.1186/s13054-016-1458-3 [Accessed 24 Jun. 2019].