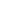
As a functional integrative medicine practitioner, you know all about homocysteine, and how this non-essential amino acid is connected to methylation and a common genetic mutation of the MTHFR SNP.
Methylenetetrahydrofolate reductase (MTHFR) is the rate-limiting enzyme in the methyl cycle, and it is encoded by the MTHFR gene. Methylenetetrahydrofolate reductase catalyzes the conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, a co-substrate for homocysteine remethylation to methionine.
As more data has come out, basing treatment off of a MTHFR mutation has become more suspect, with the majority in the medical and functional medicine space currently viewing MTHFR mutations as having minimal clinical information without factoring in other clinical and laboratory risk factors.
A homocysteine blood test is common and relatively easy to run and has been used to assess cardiovascular and cognitive health.1-2 So, should we throw homocysteine out with the proverbial MTHFR bathwater?
Not so fast.
While it's true that genetic testing has been called into question, homocysteine remains a very good proxy for how well methylation is performing, and when levels rise too high, this can provide the skilled clinician valuable information to structure their treatment around to catch cardiovascular risk that is often missed with standard measurements and labs.
Homocysteine Explained
As a sulfur-containing non-essential amino acid, homocysteine acts as an intermediate in two main pathways, the methylation cycle, and the transsulfuration pathway.4
Methylation Cycle
As a chemical pathway that occurs over a billion times a second, methylation is connected to numerous bodily functions including DNA expression, neurotransmitter production, hormone balance, and detoxification.4-5
This is accomplished through the transfer of one-carbon "methyl" groups consisting of one carbon and three hydrogens from the methyl donor SAMe to another molecule, which can turn on or off genetic processes, metabolize neurotransmitters, toxic byproducts as a part of detoxification, and much more.4
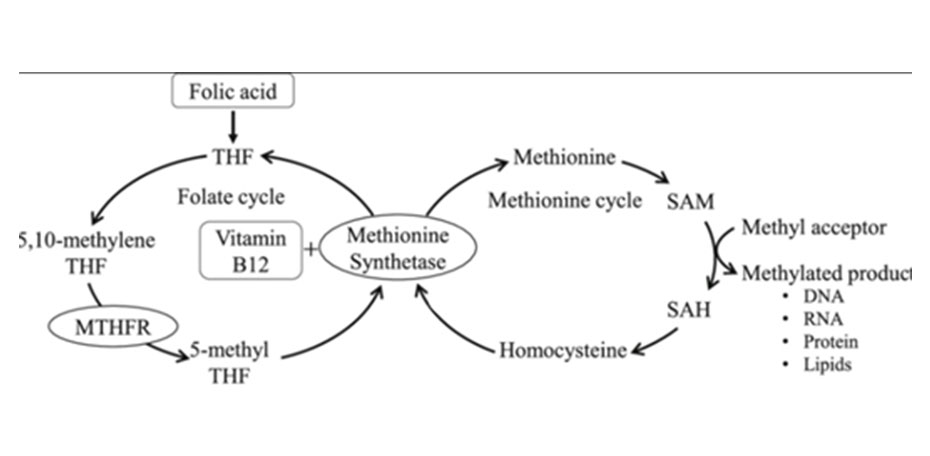
Transsulfuration Pathway
The second fate of homocysteine is through the transsulfuration pathway. This alternate pathway diverts homocysteine away from the methylation cycle towards cysteine production and ultimately the formation of our body's master antioxidant glutathione.4
During periods of increased oxidative stress, the transsulfuration pathway allows the body to quickly respond and replete glutathione stores.
Measuring Homocysteine and the Risks of Elevated Homocysteine
Homocysteine can be assessed simply through a serum test and should be considered for the assessment of cardiovascular and cognitive health. While hyperhomocysteinemia is defined as a serum level >15 umol/L, we recommend the following risk stratification:5
- <7 µmol/L - Low risk
- 7-13 µmol/L - Moderate risk
- >13 µmol/L- High risk
Currently, there is still debate over whether homocysteine is a direct risk marker or a surrogate biomarker that is not a direct contributor in the disease process. However, while many clinical trials failed to show a reduction in cardiovascular event outcomes, these trials used a secondary-prevention population currently on a polypharmacy of drugs including statins, beta-blockers, aspirin and ACEI.4 This complicates the interpretation of these results. Given this fact, as well as homocystine’s proposed mechanisms of increasing endothelial dysfunction through oxidative damage, increased platelet coagulation and vascular smooth muscle cell proliferation, we recommend that homocysteine should be assessed and treated when levels rise, to reach a level of below 7 µmol/L.5
What are the Risks of High Homocysteine?
Hyperhomocysteinemia is correlated with not only cardiovascular disease, thrombosis, and heart attacks and strokes, but also cognitive decline.2
Elevated homocysteine is also connected to B-vitamin deficiencies such as B12 and folate, and subsequent manifestations of each including pernicious anemia and certain GI disorders that result in a lowered ability to absorb micronutrients.
Additional risks associated with elevated homocysteine include:3
- Pregnancy complications (neural tube defects, preeclampsia, placental abruption)
- Down syndrome
- Osteoporosis
- Migraines
- PCOS
The Bottom Line
While elevated homocysteine is a concern, lowering the level can be accomplished through supplementing methylation cofactors including folate as 5-MTHF in order to bypass the MTHFR enzyme, as well as B12 as methylcobalamin, B6 as the active P5P form and B2.5
Betaine or trimethylglycine supplementation is also able to convert homocysteine to methionine through a non-B-vitamin-dependent step. Supplementing betaine or its precursor choline has also been shown to lower hyperhomocysteinemia and should be included in any supplement strategy targeting elevated homocysteine.5
However, merely lowering homocysteine is not the end-all-be-all of cardiovascular risk. There still needs to be a comprehensive lifestyle and dietary intervention, necessary for addressing other factors that impact methylation and transsulfuration including:
- Lowering inflammation and oxidative stress
- Improving and maximizing detoxification
- Deliberate and strategic stress management
- An individualized exercise prescription
- Targeted nutraceuticals
These strategies not only will help work around a mutated MTHFR gene that is not capable of producing enough active methylation cofactors, but also lower additional risk factors that are connected to improper methylation and elevated homocysteine levels.
References
- Diaz-Arrastia R. Homocysteine and Neurologic Disease. Archives of Neurology. 2000;57(10). doi:10.1001/archneur.57.10.1422
- Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutrition Journal. 2015;14(1). doi:10.1186/1475-2891-14-6
- Moll S, Varga EA. Homocysteine and MTHFR Mutations. Circulation. 2015;132(1). doi:10.1161/circulationaha.114.013311
- Lord R. Laboratory Evaluations for Integrative and Functional Medicine. Metametrix; 2008.
- Guilliams T. Cardiometabolic Risk Management: A Functional and Integrative Approach. 1st ed. Point Institute; 2018.

Steven Imgrund, MS, CNS
Steven Imgrund is a board-certified nutritionist and the CM Vitals Brand Manager at Lifestyle Matrix Resource Center, overseeing the marketing initiatives of their cardiometabolic product line. Steven developed his passion for nutrition and health working as personal trainer and health coach, specializing in weight loss and behavior change. Through working with hundreds of clients, Steven has seen the challenges with implementing long-term lifestyle and dietary habits. This experience has fueled his passion for functional medicine, and his desire to help health care practitioners use proven lifestyle and supplement strategies to safely address the root causes of their patients cardiometabolic issues. Steven received his Masters in Human Nutrition through Bridgeport University in 2018, and Certified Nutrition Specialist (CNS) certification in 2020.